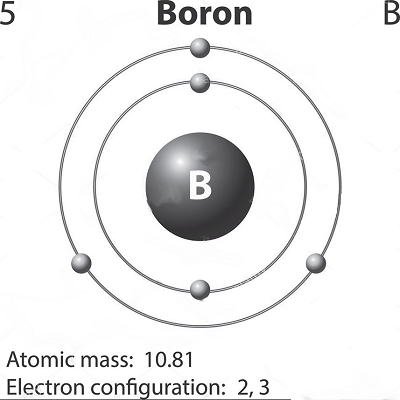
Boron, the fifth element of the Periodic Table, is one of its unsung heroes, but a hero all the same. Unfortunately, overshadowed by its high-profile neighbouring element, carbon, it does not get the attention it deserves.
Like carbon, boron too is amazingly multifaceted. And far from being boring as its name might suggest, it is highly complex in its intriguing chemical behaviour, its unusual chemical compounds, and the multiple amorphous and crystalline allotropes it can form.
Also, like carbon, boron is one of the lightest elements. But, it is extremely difficult to isolate in the pure form mainly because of its high reactivity and the fact that studying it has always proved to be too challenging. In fact, many scientists are of the view that this fascinating element has not yet been fully understood.
As for the applications of boron and its compounds, they range from being used as a flux by goldsmiths centuries ago to their modern-day use in the production of Organic Light Emitting Diodes (OLEDs) for flat-screen TVs and smartphones.
Interestingly, boron also plays a behind-the-scenes, dazzling role when it comes to gems. It gives the rare and valuable blue diamonds, including the most famous of them all—the Hope Diamond, their attractive blue colour. Blue diamonds contain trace amounts of boron trapped within the matrix of carbon atoms. And since boron absorbs low-energy red light, the white light passing through such diamonds emerges with a bluish colour.
A long history and problematic discovery
Though a naturally occurring element, boron is hardly ever found in its elemental form because of its high reactivity. It typically exists in combination with other elements in the form of boric acid or borates, compounds that are present in oceans and also in sedimentary rocks, coal, shale, and soil. Boric acid or sassolite occurs in certain volcanic springs, whereas seawater contains boric acid and borates. Large quantities of borate minerals are found as white crystalline deposits in desert-like areas.
Borax or sodium borate, the most important compound of boron, may be a common household cleaner today, but people have been using naturally occurring borax for centuries in different ways. Arabian and European goldsmiths used it as a flux, while the Chinese used it in glazes for ceramics. It is believed that ancient Egyptians could have used it for mummification.
The Tibetan lakes are considered to be the earliest source of borax. On his travels to China, Marco Polo, the famous Italian merchant, and explorer, had discovered the well-established trade of borates between Tibet and Indian cities. He is believed to be the first to import borax to Europe around the early 14th century via the historic Silk Route.
For centuries, boron proved to be a puzzling element for early researchers who often mistook it for carbon because of the similarities in some properties of these two elements. Then, in 1808, the first breakthrough was claimed to have taken place. Within a few days of each other, two research teams working on extracting boron from borate minerals—one led by Sir Humphry Davy in London and the other by Joseph Louis Gay-Lussac and Louis-Jacques Thénard in Paris—declared they had succeeded in isolating boron. As it turned out, they were mistaken. It took almost another nine decades and the efforts of another great chemist Henri Moissan to prove that what those two teams had produced was a compound containing around 60% boron. In 1892, Moissan isolated a much purer form of the element containing around 90% boron. It was not until the American chemist Ezekiel Weintraub isolated 99% pure boron in 1909 that the real breakthrough was achieved.
The name ‘Boron’ was created for the new element by Sir Humphry Davy. He coined it by combining the word ‘Borax’—since it was the mineral from which it was isolated, and the word ‘Carbon’—given its resemblance to this element.
Today, to produce a reasonably pure form of boron powder, boron oxide present in one of the four main borate minerals—tincal, kernite, colemanite, or ulexite—is heated with magnesium or aluminium flux. Through this reduction process, elemental boron powder that is around 92% pure can be produced.
Turkey, that has rich deposits of borate minerals, is the world’s largest producer of borates, exporting concentrates of borate minerals as also refined borax decahydrate, borax pentahydrate, anhydrous borax, and boric acid.
Why boron is so remarkable?
Boron, the first element of Group 13, is a metalloid or semi-metal. But, the other naturally occurring elements following below it in this group—aluminium, gallium, indium, and thallium—are all metals. Also, unlike the other elements in Group 13 that are soft and have low melting points, boron has an extremely high melting point of roughly 2076oC.
Boron is also an allotropic metalloid and can exist in several different amorphous and crystalline forms, each form having its own distinctive physical and chemical properties. Even the smallest amount of impurities can alter the structure of these forms. According to some scientists, many of the reported forms of crystalline boron could actually be boron-rich borides and not pure elemental boron.
Pure boron is either an amorphous deep brown to black powder or a dark, lustrous, hard and brittle crystalline metal. It is this brittle nature of pure elemental boron that limits its practical applications. The crystalline from of boron is the second hardest of all elements next only to the diamond form of carbon.
Boron atoms also have the special property of being able to absorb neutrons, a property that is exploited in making control rods for nuclear reactors and in instruments used for detecting neutrons.
As an alkaloid, some of boron’s properties are similar to those of metals, while others are like those of non-metals, and therefore it forms a number of interesting compounds with both metals and non-metals.
Just as carbon forms hydrocarbons, boron forms compounds called boranes. These proved to be more complex than hydrocarbons and more difficult to prepare as the pioneering chemists in this field found. Professor Herbert C. Brown, an English-born American chemist carried out further research using boranes developed earlier. In 1979, he won the Nobel Prize in Chemistry for developing new reagents containing boron for use in organic synthesis. The compounds that Dr. Brown developed, known as organoboranes, led to chemists using boron in organic reactions in a big way, and to an economical way of synthesising various molecules including some used as medications.
Ever since the discovery of carbon nanotubes, the synthesis of boron nanostructures, such as nano particles, tubes, and sheets, began to attract a lot of interest. Recent research has shown that flat boron or 2-D boron when super-cooled would be a natural, low-temperature superconductor. The researchers said flat boron may be the only such 2-D material to have such an intrinsic property.
Besides, boron is an essential micronutrient for plants, playing a crucial role in the development of their cell walls and reproductive organs, and also in nitrogen fixation and nodulation in legume crops. For some reason, a deficiency in boron results in empty pollen grains, low pollen vitality, and stunted root growth, and destroys the health and growth of crops.
Multiple and diverse modern-day uses
While traditional applications of boron, namely, the production of glass—including the tough and heat-resistant borosilicate glass, ceramics, fertilisers, detergents, and bleaches, continue to account for the use of a major quantity of the world production of boron and boron compounds, boron has also proved to be of crucial importance for satisfying a gamut of modern industrial needs.
Of the few uses that elemental boron has, its metallurgical applications are quite significant. Boron atoms can help get rid of iron and other impurities by bonding with them. And so, even a few parts per million of boron when added to steel can greatly increase its strength to around four times that of the average high-strength steel. Similarly, because of the element’s property of dissolving metal oxide films, it is added to welding fluxes. Boron also forms a critical component in Neodymium magnets—the high-strength permanent magnets whose applications range from computer hard discs and cordless tools to electric vehicles and wind turbines. Amorphous boron is used as a rocket fuel igniter and also in pyrotechnic flares to which it imparts a characteristic green colour.
Boron trichloride is used for removing nitrides, carbides, and oxides from molten metal and is therefore also used in making alloys of aluminium, magnesium, copper and zinc.
Metal borides increase conductivity and mechanical strength in powder metallurgy, increase corrosion resistance and hardness in ferrous products, and increase mechanical strength of titanium alloys used in jet frames and turbine parts.
Boron carbide, the boron-carbide ceramic, is another wonder substance. The third-hardest known material, it also has a very low density and is extremely light. It is used for making bullet proof vests, tank armour, lightweight composite materials, and abrasive and wear-resistant products. Besides, as a neutron absorber, like elemental boron it is also used in the manufacture of control rods for controlling the rate of fission in nuclear reactors.
Boric acid is used as an insecticide and as a mild antiseptic in eye drops. Some borates like disodium octaborate tetrahydrate are used as flame retardants. And Boron-10 (10B), a stable isotope of the element, is used in Boron Neutron Capture Therapy (BNCT) for treating locally invasive malignant tumours.
Recognising boron’s many potential hi-tech uses, scientists worldwide are currently researching how it can be exploited to play a critical role as a source of energy and also for energy storage and heat conservation. Not to mention the research underway on boron nanostructures. Which means, we can look forward to boron never ceasing to surprise us by serving us in astonishingly new ways even in the future.