Carbon, the wondrous element with amazing properties, forms the foundation for all known life forms on earth. Carbon atoms are the major component of many important substances and also polymers in the body including proteins, carbohydrates, fats, DNA, and RNA, and synthetic polymers such as nylon, polyethylene, polystyrene etc. Even steel, the metal that hugely influences our lives is an alloy of iron and carbon. Some of the strongest materials used today in place of metals are made of carbon fibres. Not only are we made of carbon, this remarkable element forms the backbone of the food we eat, the homes we live in, our transport systems, the things we use in daily life, global economies, and more.
Also, carbon’s potential to form countless different compounds by bonding with atoms of almost all the elements of the periodic table in numerous ways – in linear and branched chains of varying complexity, and rings, and also by way of single, double, and triple bonds – is unmatched by any other element. Let it react with hydrogen and oxygen, and you could produce myriads of compounds ranging from glucose to gasoline. In fact, an entire discipline of chemistry, organic chemistry, involves the study of just carbon compounds. To date, scientists have been able to identify around ten million carbon-containing compounds. And they’re still counting!
From soot to the stars
Known to man for millennia, from the soot formed by burning candles to the stars in the sky, carbon is present all around us. It got its name from the Latin word for coal – carbo. But, in addition to coal, that is mostly made up of carbon, and the well known allotropes diamond and graphite, carbon is present all over the earth in the form of chemical compounds such as CO2 that is a component of our atmosphere and also dissolved in the waters of lakes, rivers, and seas, calcium carbonate found in rocks of limestone, chalk, and marble, and hydrocarbons in the deposits of petroleum, and natural gas.
Revolutionary discoveries of the twentieth century
In 1946, Willard Libby, a chemistry professor at the University of Chicago, put forward an innovative method for dating organic materials by measuring their content of C-14, the radioactive isotope of carbon discovered just a few years earlier in 1940. It was widely acknowledged as a ground-breaking discovery. The celebrated British archaeologist Colin Renfrew hailed the carbon dating method as “the radiocarbon revolution”. Not surprising, since Libby’s discovery impacted not only the realm of chemistry, but also brought about radical changes in the fields of archaeology, geology, and anthropology by enabling more precise dating and understanding of historical chronologies.
When Libby began his research work in 1945, it was known that C-14 moved freely through the atmosphere, the different ecosystems, and the oceans through what is known as the carbon cycle. Libby discovered that plants absorb a specific amount of C-14 along with the carbon they absorb for photosynthesis. Realising that C-14 present in the atmosphere would somehow get into living matter, he thought that, theoretically, if the amount of this radioactive isotope in anything could be measured, one could calculate that object’s age based on the half-life of the isotope.
Using specially developed equipment, that he and his group called the ‘anti-coincidence counter’, Libby first tested samples of redwood and fir trees, whose age was already known from their annual growth rings. Next, he tested artifacts from museums including a piece of timber from the Egyptian pharaoh Senusret III’s funerary boat whose age was known from the historical record of the pharaoh’s death. The age estimated by Libby through his carbon dating technique was found to be remarkably close to the known age for all the samples he tested. Till the introduction of Libby’s innovative method of dating, archaeological artifacts, fossils, and organic remains were dated approximately, just going by the layer of the site in which they had been discovered, assuming that the site’s layers were created chronologically.
Libby’s carbon dating method invalidated many previously held beliefs, such as the notion that civilisation originated in Europe and then spread throughout the world. The new dating of man-made artifacts found in different continents helped archaeologists establish that civilisations developed independently in sites across the world. The carbon dating method can be used to date materials as old as 50,000 years. For his phenomenal discovery, Libby was awarded the Chemistry Nobel Prize in 1960.
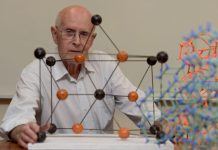
While Libby’s discovery rewrote human history and what we previously knew about the earth, the discovery of a new three-dimensional allotrope of carbon rewrote what was taught to students about carbon. Till 1985, when the British chemistry professor Harold Kroto, and the American chemistry professors Richard Smalley and Robert Curl discovered a previously unknown pure carbon molecule, C60, the world only knew of two distinct allotropes of carbon – diamond and graphite. In fact, at first a few scientists were cynical about their discovery, carbon being a diligently researched and studied element since long. They questioned how such a radically different carbon molecule could have gone undetected till then.
The three pioneering scientists named the new carbon allotrope buckminsterfullerene. They had chosen to name it after the American architect Buckminster Fuller, because it was the design of the geodesic domes he had created in the 1960s that provided the vital clue to the structure of the highly stable and neutral 60-atom carbon molecule they had discovered. They realised that the atoms of the C60 molecule could only be arranged in the form of a hollow cage. The aesthetic appeal of the structure, a truncated icosahedron with 12 pentagonal and 20 hexagonal faces, shaped like a microscopic soccer ball, led many to dub C60 as the ‘beautiful molecule’. In 1996, Curl, Kroto, and Smalley won the Nobel Prize in Chemistry for their discovery of what is now known as fullerenes. In his Nobel Prize speech, Harold Kroto, the son of German refugees, had jokingly referred to how in his school in England he felt like the odd one out as his other classmates had typical English names like Higginbottom.
Measured in nanometres, fullerenes are nanomaterials that could be carbon molecules in the shape of hollow spheres (buckyballs), tubes (nanotubes), and some other shapes as well. Buckminster fullerene, the most common naturally occurring fullerene, can be found in minute quantities of soot. Today, fullerenes have not only opened a new branch in organic chemistry they also form the vital core of nanotechnology. Fullerenes are used in novel drug delivery systems that specifically target tumour cells. Research is also being carried out on the use of carbon nanotubes for converting sea water into drinking water more efficiently than the traditional technologies.
Significant developments in the twenty-first century
Often referred to as the ‘miracle material’, graphene, the world’s thinnest material, was isolated in 2004 by researchers Andre Geim and Prof Kostya Novoselov who won the Nobel Prize in Physics for this pioneering work. Comprising a single layer of carbon atoms arranged in a hexagonal mesh, graphene is several times stronger than steel, more flexible than rubber, and conducts electricity much better than copper. The hottest carbon-related research today is centred on graphene, through mass production of this wonder material is still a challenge.
Graphene has immense potential for use in the manufacture of composite materials, photovoltaic cells, and optical electronics, and as an ultrafiltration medium and substitute for silicon in electrical systems.
One of the serious problems scientists are grappling with in the 21st century is global warming, something closely linked to the rise in atmospheric carbon. Since the Industrial Revolution, that enormously increased fuel combustion, the concentration of carbon dioxide in the atmosphere has also increased significantly. At the same time, fossil fuels, that are formed naturally over a long period of hundreds of millions of years, are being rapidly depleted.
Currently, one of the strategies suggested for reversing the global warming process and slowing down the consumption of fossil fuels is the methanol economy. This could involve using hydrogen (produced efficiently from water using alternative energy resources) for the reductive conversion of carbon dioxide to methanol, a much cleaner and cheaper fuel that could replace fossil fuels. Currently, like China, the Indian government too, is pushing for a methanol economy.
References
Nobel Laureate George A. Olah: Carbon – C&EN, 8 September 2003
Elton Santos: New form of carbon discovered that is harder than diamond but flexible as rubber – The Conversation, 24 June 2017
Royal Society of Chemistry: Periodic Table – Carbon – http://www.rsc.org/periodic-table/element/6/carbon
By Holli Riebeek: The Carbon Cycle – Earth Observatory, NASA, 16 June 2011
JRank Articles: Why Carbon is special – http://science.jrank.org/pages/1202/Carbon-Why-carbon-special.html
JRank Articles: The Chemistry of Carbon – http://science.jrank.org/pages/1201/Carbon-chemistry-carbon.html
Simon H. Friedman: The four worlds of Carbon – Nature Chemistry, 23 April 2012
Stephanie Pappas: Facts About Carbon – Livescience.com, 29 September 2017
Kevin Bullis: The methanol economy- MIT Technical Review, 2 March 2006
University of Manchester: The story of graphene – http://www.graphene.manchester.ac.uk/explore/the-story-ofgraphene/Nobelprize.org.
Nobel Media AB 2014: Sir Harold Kroto – Biographical –https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1996/kroto-bio.html
American Chemical Society National Historic Chemical Landmarks: Discovery of Fullerenes – http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/fullerenes.html