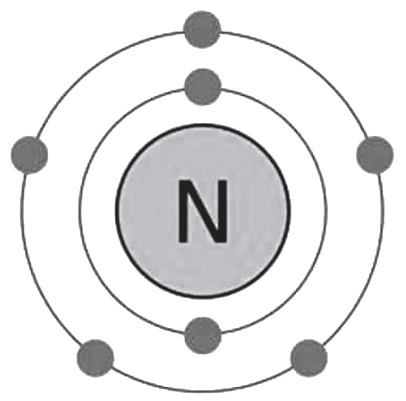
Much like Oxygen and Carbon, the seventh element of the periodic table – Nitrogen – is also a crucial, life-supporting substance. Nitrogen is a part of our DNA – our genetic code. It’s a constituent of several biologically significant molecules such as haem (in haemoglobin) and acetylcholine (the neurotransmitter in the nervous system of humans and other life forms). It is also the vital building block of amino acids that form proteins, and enzymes. It is present in our bones, blood, and tissues, constituting around 3% of the mass of the human body.
But not just for humans, nitrogen is essential for the survival, growth, and sustenance of all living things. In short, all life on earth needs nitrogen.
A colourless, odourless, non-toxic, diatomic gas that makes up around 78% of the air around us, Nitrogen is also the most abundantly available pure element on earth. However, should its concentration in the atmosphere rise way above its present levels, we would all suffocate to death.
What’s in a scientific name?
By the eighteenth century, following investigations by Carl Wilhelm Scheele, Henry Cavendish, and Joseph Priestley, scientists believed that there were two kinds of ‘airs’ – ‘fire air’ or dephlogisticated air that supported combustion (and that we now know as oxygen), and a ‘foul air’ (mostly nitrogen mixed with a small quantity of carbon dioxide, though they didn’t know this then) that was left behind once the ‘fire air’ was consumed. In 1772, a young Scottish chemist and botanist, Daniel Rutherford, carried out experiments to get rid of both oxygen and carbon dioxide from the air and found that what remained contained what he called ‘noxious’ air or ‘phlogisticated’ air. He published his findings in his doctoral thesis suggesting that air mostly consisted of this noxious gas. We now know his conclusions about the gas he had discovered were wrong, but since he was the first to publish his findings about nitrogen, he is credited with having discovered the gas.
The French scientist Lavoisier suggested the newly discovered gas be given the name ‘azote’ which he had derived from the Greek word ‘azotikos’ meaning ‘no life’. He picked the name based on the fact that the gas was mephitic since animals placed in an atmosphere that was purely made up of this gas died of asphyxiation. English scientists pointed out that with the exception of oxygen, almost all gases were mephitic and so rejected Lavoisier’s suggestion. German scientists proposed the name ‘stickstoff’ derived from the German words ‘erstricken’ (meaning suffocating) and ‘stoff’ (meaning substance). But, English scientists objected to this name as well. Then the French scientist Chaptal came up with the name ‘nitrogène’ (nitrogen) derived from the Greek words ‘nitron’ and ‘genes’ that together meant ‘nitre forming’, nitre being the old name for Potassium Nitrate from which Cavendish had successfully produced the gas. And that’s how Nitrogen got its current English name. Interestingly however, the French decided they preferred the name ‘azote’, while the Germans have stuck to ‘stickstoff’! And in English, some nitrogen compounds are indeed based on Lavoisier’s name and called azides and azo compounds.
Ammonia, one of the most abundant nitrogen-containing compounds in the atmosphere, got its name from Ammon – a powerful deity of ancient Egypt, near whose temple large salt deposits containing ammonium chloride used to be found. Ammonium chloride itself was called Sal ammoniac meaning salt of Ammon.
The most abundantly used industrial gas
Nitrogen is produced by distilling liquid air. Around 45 million tonnes of nitrogen are produced this way each year to meet the demands of a wide range of industries. Being inert, gaseous nitrogen is commonly used for providing an unreactive atmosphere for protecting reactive materials from coming in contact with oxygen, for preserving food items, and also in the electronics industry during the production of transistors and diodes. Annealing of stainless steel is also carried out in an atmosphere of nitrogen gas.
Liquid nitrogen is beneficial because of its coldness and inertness as well and is commonly used as a refrigerant. It is used for preserving sperm, eggs, and other cells for medical research and reproductive technology, for performing cryogenic surgery during which unwanted tissue is cut off by freezing it to very low temperatures, and for rapidly freezing foods so they can maintain their moisture, taste, colour, and texture.
Chemicals, pharmaceuticals, glass and ceramics, petroleum, pulp and paper, healthcare, steel, and metal refining and fabrication are some of the many industries that use nitrogen in large quantities. Nitrogen is also a key feedstock for the chemical industry, used for manufacturing hundreds of chemical compounds for diverse industries such as fertilisers, food, pharmaceuticals, nylon, dyes and explosives, automobiles, etc.
But before it can be converted to different chemicals, nitrogen must first be reacted with hydrogen to produce ammonia. Today, the Haber-Bosch process is the most economical artificial nitrogen fixation process used industrially, involving the synthesis of ammonia from nitrogen and hydrogen. Around 210 million tonnes of ammonia is manufactured each year using this process. It is first converted to nitric acid and thereafter to various other chemicals. But, ammonia is mainly used as a fertiliser, being applied directly to the soil or in the form of ammonium salts.
The Nitrogen Cycle and Nitrogen Pollution
Most of the atoms of nitrogen in the atmosphere exist as N2 – as a pair bound together by a powerful triple bond, too powerful for living organisms to break. To get access to nitrogen for their sustenance, plants and animals therefore need it to be in a reactive ‘fixed’ form, to be ‘fixed’ to elements like carbon, hydrogen, or oxygen mostly as organic nitrogen compounds. The biological process of fixing atmospheric nitrogen by converting it into ammonia is carried out by soil bacteria. Other bacteria like the ones that live symbiotically inside nodules on the roots of plants such as beans and peas, convert the ammonia into complex nitrogen compounds like amino acids and proteins.
The other source of naturally occurring reactive nitrogen is due to lightning. The energy it generates converts oxygen and nitrogen to nitric oxide (NO). This is oxidised to nitrogen dioxide (NO2), and then to nitric acid that is carried from the atmosphere to the ground by rain, snow, or other forms of deposition. Lightning plays an important role in nitrogen fixation in areas where there’s a scarcity of nitrogen-fixing plants.
‘Nitrogen fixation’ is actually Nature’s way of preparing fertilisers in the soil for nurturing plants directly and all life on earth indirectly. Plants are the first to feed on the nitrogen compounds. Animals get their requirement of these by feeding on plants. And then humans get theirs from the plants and animals they eat. Nitrogen compounds return to the soil in the form of human and animal waste products. Bacteria in the soil then convert these nitrogen compounds back to nitrogen gas, which is released into the atmosphere.
Since long now, to feed a rapidly growing world population, crop yields are being boosted by adding fertilisers to the soil. However, excessive use of fertilisers in agriculture destroys soil health rather than making it more fertile, besides adversely affecting the environment, aquatic life, and human health as well. The excess fertiliser is converted by soil bacteria into nitrates that then get leached into the ground water or get washed out of the soil into rivers and lakes. Algae in the water bodies feed on the nitrates and grow rapidly, reducing the oxygen available in the water for aquatic flora and fauna. The pollution of ground and surface water leads to domestic water supply too, having dangerously high nitrate levels. Consuming fertiliser-polluted water causes various types of cancer, reproductive problems, hypertension, and ‘blue baby disease’ in infants. Babies fed formula prepared with nitrate-rich water, develop a blue-grey colouration of the skin due to decreased oxygenation of their blood.
Overuse of fertilisers also results in increased nitrogen emissions such as ammonia, nitrogen oxide, and nitrous oxides. These nitrogenous gases lead to respiratory problems in humans, and also play a prominent role in global climate change, nitrous oxide being one of the more potent greenhouse gases.
But, despite its disastrous consequences, nitrogen pollution is not getting as much attention as carbon pollution. It is estimated that in Europe alone, the environmental and human health costs of nitrogen pollution could be around €70-320 billion per year. The nitrate levels in the waterways of most industrialised nations are on the rise. In the rivers in the northeastern United States and many countries in Europe, nitrate concentrations have increased 10 to 15 times in the last 100 years. Also, Oxygen-starved areas or ‘dead zones’ have been found in the Gulf of Mexico, the Baltic Sea, the Adriatic Sea, the Gulf of Thailand, the Yellow Sea, and the Chesapeake Bay.
Reducing our nitrogen footprint
Clearly, it’s now time to take a closer look at our nitrogen footprint too, that is, the amount of nitrogen released into the environment by different human activities. The best part is that reducing our nitrogen footprint will also reduce our carbon footprint. According to many experts, the solution to curbing the excessive use of nitrogen fertilisers could be sustainable agriculture, popularisation of organic farming, and educating farmers about the environmental issues arising out of uncontrolled use of fertilisers.
References
1. Agata Blaszczak-Boxe: Facts about Nitrogen – LiveScience.com, 27 September, 2017, https://www.livescience.com/28726-nitrogen.html
2. R. Thomas Sanderson: Nitrogen – Encyclopaedia Britannica, 27 April, 2018, https://www.britannica.com/science/nitrogen
3. John Emsley: Nature’s Building Blocks: An A-Z Guide to the Elements – Oxford University Press, 2011
4. Universal Industrial Gases Inc: Nitrogen (N2) Properties, Uses and Applications – Nitrogen Gas and Liquid Nitrogen – http://www.uigi.com/nitrogen.html
5. Royal Society of Chemistry: Periodic Table – Nitrogen – http://www.rsc.org/periodic-table/element/7/nitrogen
6. Yale University: The world’s nitrogen fixation, explained – ScienceDaily, 23 September 2015, www.sciencedaily.com/releases/2015/09/150923133513.htm
7. Ee Ling Ng, Deli Chen, Robert Edis: Nitrogen pollution: the forgotten element of climate change – The Conversation, 5 December, 2016.