Abstract
The contact process uses vanadium pentoxide catalyst to increase the rate of reaction of producing sulphuric acid. Sulphuric acid plants periodically require either catalyst replacement or make-up in order to cope with the sieving losses so that the process efficiency is sustained for the subsequent production run. The spent catalyst is considered as hazardous solid waste and cannot be discarded untreated owing to the presence of considerable composition of vanadium and other associated metals. Because of significant environment implications of spent catalyst wastes, it is imperative to recover valuable metals present in the catalyst. The recovery of precious materials or metals from waste will not only help in mitigating environment problem due to metal pollution but also is a source of additional revenue generation through recycling of precious metal. Recovery of vanadium from spent industrial catalysts is being widely practiced worldwide.
However, due to diversity in characteristics, many of these extraction methods vary in terms of their effectiveness as well as economic feasibility. The recovery of vanadium from spent vanadium is being performed in this project work by making use of catalyst sample obtained from M/s. Fertilisers and Chemicals Travancore Ltd. A three step pathway comprising of acid leaching and oxidation followed by precipitation was employed. The iteration of various parameters associated with the process was carried out and their effect on the efficiency of the overall process was studied. The most optimum process route for the recovery operation was concluded after arriving at the best values of the various parameters as depicted by the lab experiments. The chemical analysis of the product was carried out to deduce the efficiency of the overall recovery process.
Introduction
Vanadium finds extensive use in various catalysts applications for numerous industrial processes. The oxides of vanadium are catalysts especially in vapour-phase reductions. Vanadium compounds have been found effective for catalyzing both organic oxidation and reduction. Major uses of vanadium as oxide are in the production of oxidation catalysts for the manufacturing of sulphuric acid, in petroleum refinery for catalytic cracking of heavy oil and in many industrial processes. Vanadium pentoxide (V2O5) is the most commonly produced compound of vanadium which finds application in manufacturing of detergents. However mostly it is used as an industrial catalyst in manufacturing of sulphuric acid through Double Contact Double Absorption (DCDA) technique.
Vanadium pentoxide catalyst (shall be henceforth referred as catalyst) melts at 690°C and decomposes at 1750°C. The role of this catalyst, in contact process for manufacturing of sulphuric acid, is to support the conversion of sulphur dioxide gas to sulphur trioxide for its subsequent reaction with water to form sulphuric acid vide the following reaction.
2SO2(g)+O2((g)→2SO3((g)———————————- (1)
The reaction is favoured by temperature condition of 440oC and is carried out in equipment called converter which pivotal to contact process.
The converter comprises of four beds packed with catalyst which comes in contact with the sulphur dioxide gas. The gas flows in series from one bed to another with heat recovery devises and absorption towers for temperature control across the catalyst beds and production of acid respectively.
The life of catalyst varies from 5 to 10 years depending upon the impurities in the feed and number of cycles used. The catalyst is subjected to temperature in the range of 440-590°C and pressure around 350 mmWC continuously throughout the production year.
The challenging conditions results in powdering or crushing of the catalyst. The catalyst is discharged after its campaign period, sieved and recharged into the converter reactor with additional make-up for the screening losses. The losses can vary from 10% to 20% of the total catalyst volume. The vanadium content and crushing strength of the used catalyst analyzed to assess the overall health of the catalyst charge.
The spent catalyst is disposed either through the manufacturer or certified hazardous waste handling and disposing agencies. Disposal of the spent catalyst raises the question of its impact on the environment. Due to heavy metal content, spent catalyst is categorized as hazardous waste and therefore need to be processed for the total metal recovery prior to its safe disposal. Recycling of spent catalyst is also important from environmental and economic perspective. One of the major benefits is a significant energy saving using recycled materials compared to virgin materials. It also helps in waste management by limiting the amount of waste on secured landfills.
The increased demand of vanadium by the industry has put extra pressure for the production of metal. This has encouraged researchers to look for alternative or secondary sources such as industrial or electronic wastes, spent catalysts and other by-products. Among secondary resources, the metal recovery from spent catalyst is gaining interest due to both, its hazardous nature and stringent regulations associated with disposal methods.
Main techniques for the separation and purification of vanadium in spent catalyst leach solutions are precipitation, carbon adsorption, ion exchange and solvent extraction.
The loading capacities of activated carbon for vanadium are relatively low, resulting in no industrial application. The scale of application of ion exchange in industry is limited owing to high cost of exchangers although it can be used to separate vanadium from other associated impurities almost completely thereby producing high purity products. Solvent extraction is the well-established cost effective operation for purification of vanadium in their aqueous solutions. Solvent extraction technique is one of the most attractive alternatives for this purpose. It has certain inherent advantages such as ease of continuous operation, high throughputs and improved economics coupled with flexibility of handling a variety of metal solutions from diverse sources. A significant effort has been made to recover vanadium from aqueous solutions by solvent extraction technique using various extractants.
However, the problem of emulsion formation frequently prevents quantitative phase separation. Poor selectivity of extractants also raises issues occasionally. Even though the efficiency of vanadium recovery is lower by precipitation method in comparison with solvent extraction technique, the relatively weighty advantages offered by the former such as low cost and simple operation and humble infrastructural requirements are admirable. All these advantages were considered in selecting the precipitation technique as the favourite for this experiment to recover vanadium from spent catalyst.
Recovery of vanadium from the spent catalyst was successfully carried out in this project work. The project has the objective to study the possibilities for the recovery of vanadium pentoxide by dissolving the spent catalyst with suitable acid media and oxidize the vanadium to higher oxidation state then precipitate it directly by converting the vanadium into simple vanadate compounds which is insoluble in the pH adjusted solution. Alkaline leaching is more selective for iron but dissolves some silica and is costlier in terms of reagent.
The employed approach is three step pathway comprising of leaching, oxidation followed by precipitation. Sulphuric acid solution was used to leach the ground sample at a controlled temperature condition. The leached solution was oxidized with potassium permanganate. This is followed by precipitation using Sodium carbonate solution. The project progressed by varying the different parameters associated with process and concluding the effect of each variation upon the overall efficiency of the process and thereby selective the most optimum pathway for the recovery operation through precipitation method. The parameters hence varied included the following: Leaching agent, leaching agent concentration, leaching temperature, time of leaching & S/L ratio.
The optimum leachate was oxidized using potassium permanganate and precipitated using sodium carbonate solution. The efficiency of leaching operation, precipitation and thereby the overall efficiency of recovery operation was deduced through chemical analysis techniques at various stages of the process. Both titrimetric technique and atomic absorption spectroscopy was employed for these analysis.
A lab-scale economic feasibility of the operation was carried out using the result of the separation process. The commercial price of sodium vanadate available in the market was studied to conclude the economic viability of the process. Finally, a schematic design of pilot plant suitable for the subject recovery operation is proposed. Batch operation is suggested due to the nature of huge content of suspended solid particles involved in the process.
Materials and Methods
Sample
The spent vanadium pentoxide catalyst sample was obtained from sulphuric acid plant in Udyogamandal division of M/s. Fertilizers And Chemicals Travancore Ltd (FACT). The sampling was done from the spent catalyst after screening and disposal. The sample belonging to the genre of ribbed ring type with 12 x 04 mm dimension had the following properties upon its physico-chemical analysis:
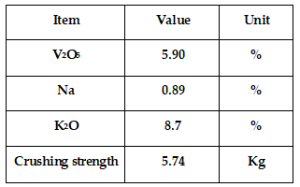
The average crushing strength of 5.74 Kg in comparison with the desirable average of above 10 Kg and vanadium composition below nominal value of 6% were the reasons that contributed in replacement of this catalyst. Nearly 1 kilogram of the catalyst sample was ground to a fine powder using pestle & morta arrangement. The powder was then dried for one hour in electric oven at 110oC. The dried powder was then sieved to obtain uniform powder of 100 mesh size. The reduction in size of the catalyst particles and its uniformity directly impacts the efficiency of leaching operation.
Chemistry of Process
The recovery process progresses in three stages namely leaching of the catalyst, oxidation of the leachate and precipitation of vanadium from the oxidized solution. The chemical reactions governing these stages are as follows:
Leaching
Vanadium pentoxide reacts with sulphuric acid to produce vanadyl sulphate, water and oxygen. The reaction slowly proceeds in a boiling solution as follows:
2V2O5 + 4H2O4→ 4VOSO4 + 4H2O + O2 ————-(2)
Oxidation
Oxidation of vanadyl sulphate by Hydrogen peroxide involves multiple reactions at neutral pH conditions. The primary reaction is oxidation of V(IV) to V(V) using 0.5 equivalent of H2O2.
Precipitation
Vanadium pentoxide reacts with sodium carbonate to form sodium vanadate as follows:
V2O5 + Na2CO3→ 2NaVO3 + CO2 ——————— (3)
Experiment
The experiment was carried out in a 500 ml beaker. The agitation necessary for the experiment was provided by magnetic stirrer integrated with thermostat for temperature control.
Leaching
The first step of the experiment was to carry out the leaching of ground and dried catalyst powder for recovering the vanadium content of the catalyst. However, there were several variables to be fixed before completion of this stage. These included the selection of leaching agent, leaching temperature, concentration of leaching agent, stirring time S/L ratio etc. The knowledge on the subject gathered from literature review was helpful in fixing some preliminary parameters while one of the parameter was iterated at a time to conclude the final value of all the parameters.
Selection of Leaching Agent
25 grams of the powdered & dried sample was leached using three of the most commonly available mineral acids namely sulphuric acid, hydrochloric acid & nitric acid. The concentration of all three acids were maintained at 20% (V/V) sulphuric acid solution. Leaching time of 30 minutes was provided. Temperature was maintained at 60oC. 125 ml of leaching acid was used so as to maintain an S/L ratio (V/V) of 1:5.
The leachate was filtered using vacuum filter unit and the clear solution free from turbidity was subjected to atomic absorption spectrophotometer for analyzing the vanadium composition in each leachate sample. The result was used to plot a vanadium concentration chart for each acid as given below:

The best leaching result was obtained when nitric acid was used as the leaching agent. However, the result provided by sulphuric acid was very close to this and hence this was selected as the optimum leaching agent due to the high difference in the cost of the two.
Moreover, the leaching using nitric acid was accompanied by emanation of fumes. Hence the subsequent iterations of leaching were done with sulphuric acid as the leaching agent.
Selection of acid concentration
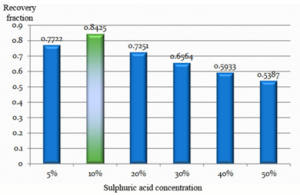
25 grams of the powdered and dried sample was leached with 125 ml of sulphuric acid. The concentration of acid was varied from 5 to 50% (V/V) keeping all the remaining parameters constant. The filtered leachate was subjected to atomic absorption spectrophotometer for analyzing its vanadium composition. The result was used to plot a vanadium concentration chart for each concentration of sulphuric acid as given below:
Selection of Leaching Time
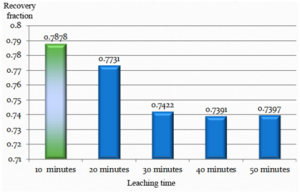
25 grams of the powdered and dried sample was leached with 125 ml of 10% (V/V) sulphuric acid. The leaching time was varied from 10 minutes to 50 minutes keeping the remaining parameters constant. The filtered leachate was subjected to atomic absorption spectrophotometer for analyzing its vanadium composition. The result was used to plot a vanadium concentration chart for different leaching times as follows.
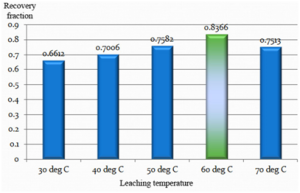
Selection of Leaching Temperature
25 grams of the powdered & dried sample was leached with 125 ml of 10% (V/V) sulphuric acid for 10 minutes. The leaching temperature was varied from 30oC to 70oC maintaining an S/L ratio of 1:5. The filtered leachate was subjected to atomic absorption spectrophotometer for analyzing its vanadium composition. The result was used to plot a vanadium concentration chart for different leaching temperatures as follows.
Selection of S/L Ratio
During all the above experiments a fixed S/L ratio of 1:5 was maintained. This was varied to 1:3 on the lower side and 1:10 on the higher side keeping all the remaining parameters constant. The variation of S/L ratio was found to have negligible impact on the leaching efficiency. Lower S/L ratio posed difficulty in stirring operation and higher S/L ratio was uneconomical due to want of higher acid quantity. Hence the optimum S/L ratio of 1:5 was selected as the optimum parameter.
Optimum Leaching
The final results of iteration were used to conclude the most optimum path for final leaching. 225 ml 10 % (V/V) sulphuric acid was used as the leaching agent against 45 grams of powdered & dried catalyst. Temperature of 60oC was maintained for leaching time of 10 minutes with an S/L ratio of 1:5. The leachate was filtered with a vacuum filter unit. Agitation was provided by magnetic stirrer and temperature was adjusted using rheostat. The filtered leachate was diluted and excess 10 % (V/V) sulphuric acid was added to it. This solution was titrated against 0.1 N ferrous ammonium sulphate (FAS) with barium diphenylamine sulphonate as the indicator.
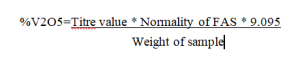
The calculation yielded a V2O5 concentration of 5.1% (w/w) in the final leachate.
Oxidation
Out of 225 ml of final filtered leachate, 150 ml was oxidized with 01 normal potassium permanganate (KMnO4) solution. Completion of oxidation was indicated by change of green tinge of the solution to golden yellow colour. This converted all vanadium in them +4 & +3 oxidation states into pentavalent state. 30% Hydrogen peroxide (H2O2) was also considered as an alternate option for use as oxidizing agent. However, the latter option was dropped due to the high cost of hydrogen peroxide in comparison with that of potassium permanganate solution and also on account of need for refrigeration to store hydrogen peroxide in healthy condition for subsequent usage.
Precipitation
The oxidized solution was precipitated by addition of 01 mol/litre solution of sodium carbonate. The pH of the solution was raised above 7.0 with the help of an electronic pH meter. The change of clear golden yellow solution to turbid yellow color solution indicates precipitation of vanadium as sodium vanadate. The precipitate was filtered using vacuum filter unit and was subsequently dried in electric oven at 105oC for 2 hours. The dried precipitate was weighed with an electronic weighing machine.
Sample Analysis
The analysis of vanadium content of the sample was experimentally carried out to determine its exact value for the sample under investigation. A fusion mixture comprising of 1 gram of powdered and dried catalyst sample mixed with 5 grams each of sodium carbonate and potassium carbonate was made and placed in a platinum crucible. This was topped up with additional 1 gram each of sodium carbonate and potassium carbonate on top of the mixture. The crucible containing the fusion mixture was placed in oven at 1200oC for 2 hours. The fused mixture was dissolved in 8% (V/V) sulphuric acid and oxidized with 01 N KMnO4 solution. This solution was titrated against 0.1 N ferrous ammonium sulphate (FAS) with barium diphenylamine sulphonate as the indicator. Colour change from violet to greenish blue indicated completion of titration.

The calculation yielded a V2O5 concentration of 5.9 % in the selected catalyst sample.
Results and Discussions
Results of experiment
The recovery of vanadium from spent vanadium pentoxide catalyst was performed through a three step pathway. The first step involved the leaching of powdered & dried catalyst using a suitable leaching agent. The optimum parameters for leaching operation were concluded by varying their values within a range and then fixing the ones that yielded the best leaching results. The results are as follows:
Leaching agent
Out of the three mineral acids used for conducting the leaching of catalyst sample namely HNO3, HCl & H2SO4, nitric acid yielded the best leaching result with a recovery of 84.8%. This was closely followed by sulphuric acid with a recovery efficiency of 84.6%. However, the result weighed more in favor of the latter due to its attractively lower cost and non-generation of hazardous fumes during the leaching operation. Hence sulphuric acid was selected as the final leaching agent.
Leaching acid concentration
The concentration of sulphuric acid was varied from 5 to 50 %. The best leaching efficiency of 84.25 % was obtained with an acid concentration of 10 %. Efficiency decreased when acid concentration moved in either direction.
Leaching time
The time of leaching was varied from 10 to 50 minutes. The best leaching efficiency of 78.78% was obtained with a leaching time of 10 minutes. The option below this was not considered due to difficulty in attainment of leaching temperature within shorter interval.
Leaching temperature
The leaching temperature was varied between 30oC to 70oC. The best leaching efficiency of 83.66% was obtained at the temperature of 60oC. The results reduced considerably in either direction.
S/L ratio
Three ratio were used namely 1:3, 1:5 & 1:10. The ratio 1:5 was selected as the optimum one since lower ratio posed difficulty in stirring operation due to the nature of the reaction mixture with significant amount of non-reactive suspended solid particles. Higher ratio was ineffective w.r.t higher reagent cost.
The optimum leaching was carried out with the above parameters and the result was analyzed to arrive at the overall efficiency for leaching obtained in the project work.
V2O5 content in the catalyst sample: 5.9 % (w/w)
V2O5 content in the leachate solution: 5.1 % (w/w)
Efficiency of optimum leaching : 86.44 %
The oxidized and precipitated vanadium (as sodium vanadate) was filtered, dried and weighed. The calculations projected the overall efficiency of the recovery operation (Appendix I)
The overall efficiency of recovery : 72.67 %
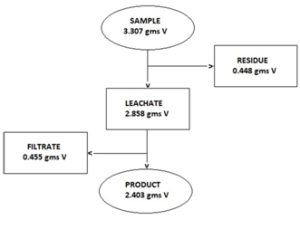
Vanadium Balance in the Process
Basis: 100 grams of catalyst sample
Economics of Recovery Operation
A laboratory scale economic feasibility assessment of the recovery process was done in the following manner. This may not be linearly extrapolated to arrive at industrial scale viability. The process is suitable for batch-wise operation due to the nature of suspended silicate material that will result in choking problems during a continuous operation.
Basis: 100 grams of vanadium pentoxide catalyst (3.307 gms V) Cost of reagents:
10 % sulphuric acid, 500 ml : Rs. 3/-
KMnO4, 5 grams : Rs. 2/-
Sodium carbonate, 1000 grams : Rs. 45/-
Total cost of reagents : Rs. 50/-
Power : Rs. 45/-
Miscellaneous (Filter paper, water etc) : Rs. 5-
Overhead, 10% : Rs. 10/-
Total cost for 3.307 gms V : Rs. 110/-
Unit cost of handling 1 gram V : Rs. 33.3
Market price of sodium metavanadate, 500 gm : Rs. 24,472/- (As on 01.04.2018)
Unit price of 1 gram sodium metavanadate : Rs 48.9
It could be inferred that during the lab-scale recovery process carried out for vanadium pentoxide catalyst, each gram of vanadium recovered as product in the form of sodium vanadate fetched a contribution of Rs.15.6
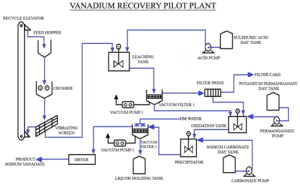
Pilot Plant for the Process
A pilot plant design is proposed to suite the process detailed in the project work. Continuous operation of plant is not feasible due to the bound choking and clogging problems that would accompany while handling the quantum of suspended solid silicate matter. Hence batch operation is proposed.
Conclusions
Summary of the Project
Spent vanadium pentoxide catalyst sample from sulphuric acid manufacturing industry was subjected to a vanadium recovery procedure comprising of acid leaching, oxidation and precipitation. Sulphuric acid was selected as the leaching agent. The leachate was oxidized using potassium permanganate solution to bring all vanadium to its pentavalent state. The oxidized vanadium was precipitated by addition of sodium carbonate solution. The vanadium precipitated in the form of sodium vanadate was dried to obtain the end product. The vanadium content in process intermediates were assessed using both analytical techniques as well as by subjecting their samples to atomic absorption spectrophotometer. The efficiency of leaching as well as the overall efficiency of the proposed recovery process was concluded and is as given in the following section.
Summary of Results
- Even though nitric acid yielded better leaching result in comparison with sulphuric acid and hydrochloric acid, sulphuric acid was selected as the suitable leaching agent due to its cost effectiveness and also clean nature of leaching without emanation of fumes.
- 10 % (V/V) concentration of sulphuric acid provided the best leaching result.
- 60oC proved to be the optimum leaching temperature.
- 10 minutes yielded the best leaching result. Interval below this was not feasible due to lag in attainment of favorable leaching temperature.
- S/L ratio of 1:5 was found to be optimum.
- 1 N potassium permanganate provided desired oxidation of vanadium into its pentavalent state.
- 1 mol/ltr solution of sodium carbonate was used for precipitating the product vanadium in the form of sodium vanadate
- The efficiency of leaching resulted as 86.44 %.
- The efficiency of overall recovery of vanadium resulted as 72.67 %.
- A laboratory scale economic feasibility of the recovery operation was assessed. It was found that every gram of recovered sodium vanadate yielded a net positive contribution in the project.
- A pilot plant design is proposed to suite the process.
There exists further scope for improving upon the efficiency of proposed vanadium recovery process by betterment of leaching efficiency with increase in the number of stages. Moreover, the vanadium in the final filtrate after precipitation remains to be extracted with suitable alternative method. However, the feasibility of further treatment of this filtrate is something that remains to be explored.
Result Calculations
| Weight of catalyst sample for final leaching | :45 grams |
| Volume of leaching acid | :225ml |
| Volume of leachate | :150ml |
| Final weight of the catalyst sample in solution | :28.902 grams* |
| Vanadium composition in sample | :5.9% |
| V2O5 content in the sample | :1.705 grams |
| Weight of precipitated sodium vanadate | :1.661grams |
| Molecular weight of V2O5 | :182 |
| Molecular weight of NaVO3 | :122 |
| V in sample | :0.9555 gram |
| V in precipitate | :0.6944 gram |
| Efficiency of vanadium recovery | :72.67% |
* Two 5 ml samples were drawn from the above leachate for intermediate analysis.
Acknowledgement
I express my gratefulness to M/s. Fertilisers and Chemical Travancore Ltd (FACT), my parent organization, for facilitating the resources for accomplishing the research works related to the project.
I express my gratitude beyond words to the following persons for their valued support and indispensable role in the progress & successful completion of this project work:
- Shri T C Abraham, Senior Chemist (Quality Control), FACT Ltd.
- Shri. Selvaraj K S, Deputy Manager-SG (Quality Control), FACT Ltd.
- Shri Karunakaran V, Deputy Manager (Production), FACT Ltd.
- Dr. G Madhu, HoD, Chemical Engg Dept, Cochin University of Science & Technology
- Shri Jamal V, Senior Manager (Udyogamandal Acid Plant) FACT Ltd.
References
- Archana Saily Painuly, Separation and recovery of vanadium from spent vanadium pentaoxide catalyst by Cyanex 272, Painuly Environmental Systems Research (2015) 4:7, DOI 10.1186/s40068-015-0032-3
- Khalid M. Mousa and Safa K. Kouba, Recovery of vanadium pentoxide from spent catalyst used in the manufacture of sulphuric acid, Iraqi Journal of Chemical and Petroleum Engineering, Vol.11 No.2 (June2010) 49-54, ISSN: 1997-4884
- Sisi Long, QimingFeng, Guofan Zhang, Dongsheng He, Recovery of vanadium from alkaline leaching solution from roasted stone coal, doi: 10.2306/scienceasia1513- 1874.2014.40.069, ScienceAsia 40 (2014): 69–72
- H.N.Dash, D. Rout and B.B.Kar, Extraction of Vanadium from Vanadium bearing Spent Catalyst through heat treatment method, International Journal of Innovative Research in Science, Engineering and Technology Volume 2, Issue 7, July 2013
- S. Khorfan, A.Wahoud and Y. Reda, Recovery of vanadium pentoxide from spent catalyst used in the manufacture of sulphuric acid, periodica polytechnic serchemeng. VOL. 45, NO. 2, PP. 131–137 (2001)
- Falak.O.Abas, Vanadium Oxide Recovery from Spent Catalysts by Chemical Leaching, Eng.&Tech.,Vol.26, No2, 2008
- Helena I. Gomes, Ashley Jones, Mike Rogerson, Gillian M. Greenway, Diego Fernandez Lisbona, Ian T. Burke, William M. Mayes, Removal and recovery of vanadium from alkaline steel slag leachates with anion exchange resins, Journal of Environmental Management 187 (2017) 384e392, Elsevier Ltd. 0301-4797/© 2016
- Alma Rojas-Rodriguez & Nestor Lopez-Castillo, Alkali process to removal of vanadium and molybdenum from spent hydrodesulfurization catalyst, International Journal of Advances in Science Engineering and Technology, ISSN: 2321-9009, Vol-4, Iss-3, Spl. Issue-2 Sep.-2016
- Hugo Calderón and Diana Endara, Recovery of Vanadium from Acid and Basic Leach Solutions of Spent Vanadium Pentoxide Catalysts, Journal of Geological Resource and Engineering 4 (2015) 213-218 doi:10.17265/2328-2193/2015.04.006
- J.G.Sunderland,Therecovery andrecyclingofvanadiumandnickelproduct from the combustion residues of orimulsion and other fuels, Project number PE-5426- 92, 1995.
- Matthys Karel Gerhardus Vermaak, Vanadium recovery in the electro aluminothermic production of ferrovanadium, Journal from Department of Metallurgy, University of Pretoria, January 2000
- Thomas G. Goonan, Vanadium Recycling in the United States in 2004, U.S. Geological survey circular 1196–S Version 1.1, October 6, 2011
- Xingbin Li, Chang Wei, Zhigan Deng, Cunxiong Li, Gang Fan, Minting Li and Hui Huang, Recovery of Vanadium from H2SO4-HF Acidic Leaching Solution of Black Shale by Solvent Extraction and Precipitation, Metals 2016, 6, 63; doi:10.3390/met6030063
- Central Pollution Control Board (India), Standard Operating Procedure and Checklist of Minimal Requisite Facilities for utilization of hazardous waste under Rule 9 of the Hazardous and Other Wastes (Management and Transboundary movement) Rules, 2016
- Mohammadreza Tavakolikhaledi, Vanadium: leaching and solvent extraction, thesis submitted at University of British Columbia, Vancouvar, May 2014.
































