Differential Scanning Calorimetry (DSC) is frequently used to measure the enthalpy and rate of chemical reactions. These measurements are important for identification, designing processes and assessing potential hazards in chemical synthesis and decomposition reactions as well as for thermodynamic and kinetic calculations. Whether a product is thermally unstable or represents a potential explosion hazard (thermal runaway) must be determined at a very early stage of process design.
Introduction
Assessing the potential hazards for chemical reactions is crucial. An important concern is the potential danger that could arise through an uncontrolled temperature increase while handling, transporting and storage of materials. The risk of a thermal runaway and an explosion is significant if
- The reaction enthalpy is large and exothermic,
- The rate of increase of temperature is high or even self-accelerating,
Gaseous products or vapors produced,whether through decomposition or evaporation - The reactor system cannot withstand high pressure and/or high temperatures,
- Subsequent complications such as fires or environmental pollution caused.
In such risk analyses, the determination of the reaction enthalpy or a decomposition enthalpy is often the starting point for further investigations. The focus is naturally on high risks.
Past experience shown that all chemicals and reactions must be investigated, not just those that are thought to be potentially dangerous. A rapid screening method is very desirable in view of the fact that the large number of tests have to be perform. DSC has proven to be an ideal technique for providing the necessary information at an early stage of the chemical product development. The DSC results help one to assess the risks with regard to thermal stability as shown schematically in Figure 1.
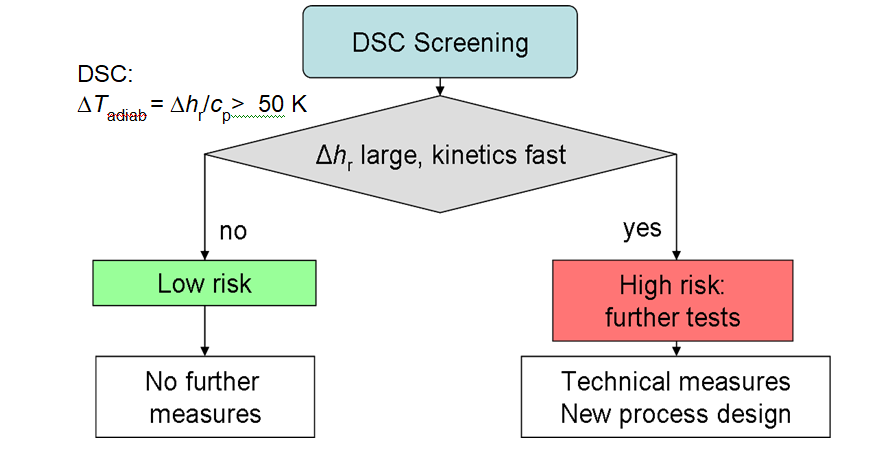
DSC measures reaction enthalpy (Δhr) and specific heat capacity (Cp). These two quantities can be used to estimate the highest temperature, ΔTad, that could reach in of a thermal runaway: ΔTad=Δhr/Cp
If the adiabatic temperature increase is more than50 K, the situation is potentially dangerous. If the increase is 200 K, the situation becomes extremely dangerous. The boundaries of course depend on the particular circumstances. In fact,the rate of production of heat is just as important as the reaction enthalpy itself.If the heat exchange with the surrounding is inadequate, dangerous adiabatic behavior may occur. This is particularly critical with fast reactions.
Example 1: The adiabatic course of a reaction can be calculated using data from a dynamic DSC experiment. Kinetic evaluation of one or more DSC measurements allows reaction behavior to be predict with regard to time and temperature using nth order kinetics.
The time to maximum reaction rate (TMR) can also be determined with relatively simple calculations. For example in case of decomposition of Benzoyl Peroxide, as shown in Figure 2a, the thermogram shows a dynamic DSC measurement of the thermally induced decomposition reaction at a heating rate of10 K/min. The measurement curve shows that the reaction begins at about 80 °C. It takes place over a temperature range of 80 K and generates an exothermic enthalpy of reaction of 1365 J/g. Assuming a constant specific heat capacity of 2 J/(g K), the adiabatic temperature increase, ΔTad, is calculated to be 682.5 °C – a value that is so high that the material would completely decompose.
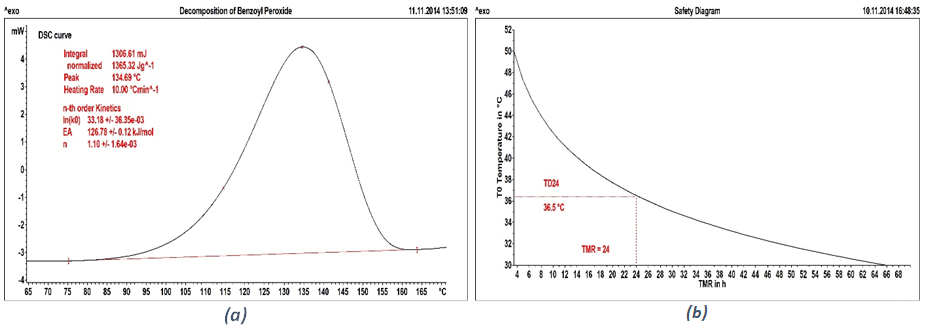
The graph in Figure 2(b)shows the relationship between TMR and the process temperature, T0. For situations with temperatures above 36.5 °C, the process can be consider as inherently unsafe. It is therefore not acceptable in a production environment and needs changes or are design.
Example 2: The conversion of a reaction can be describe as a function of temperature and time with the aid of just a few DSC measurements followed by evaluation using Model Free Kinetic (MFK). The results allow predictions to make about the long-term behavior (e.g. storage conditions) or short-term behavior (explosions) of substances.
For example, in order to assure safety in chemical plants, a thorough understanding of the thermal hazard of materials like the potential explosive 2-Nitrophenol is essential.
For safety investigations dynamic DSC measurements at four different heating rates were perform. These are displayed in the top left quadrant of the Figure 3. The advanced model free kinetics software calculates the conversion (top right) and displays the activation energy as a function of the reaction conversion (conversion plot, bottom right).
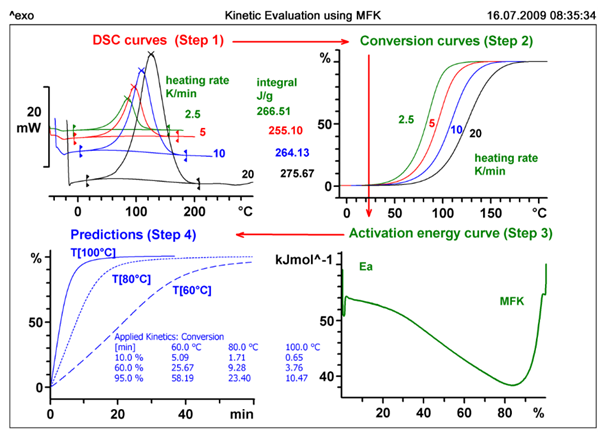
Model-free kinetics assumes that the activation energy does not remain constant during a reaction but changes. The conversion-dependent activation energy can now be used to make predictions, for example for the conversion of a reaction at a particular temperature as a function of time. This is done on the bottom left for three different temperatures between 100 and 150 °C.
The predictions should be checked by performing a real measurement under the appropriate conditions.
Conclusion
Mettler Toledo DSC measurement along with STARe software tools provides best solution for safety analysis. The risk of a reaction runaway can, in principle estimated from DSC measurements of the adiabatic temperature increase, ΔTad, and the Time to Maximum Rate (TMR) using nth order kinetics. Advanced model free kinetics allows the prediction of the reaction course at almost any temperature.
For more information:
Visit: www.mt.com/ta-safety
www.mt.com/ta
Email: sales.mtin@mt.com
Toll-free: 1800 22 8884 and 1800 1028 460
































